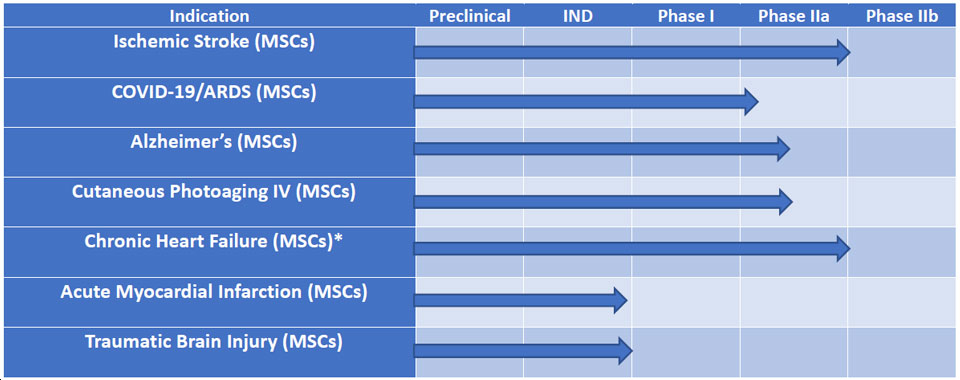
Clinical Pipeline
- Home
- Clinical Pipeline
Strategy
In order to produce empirical evidence on the translational value of progenitor cell-based therapies for illnesses and disorders for which there are presently no viable therapeutic alternatives, Sanostem carries out substantial pre-clinical and clinical research in collaboration with partners.
Sanostem has collaborations with researchers and physicians from prestigious universities, top research institutes, and recognized healthcare facilities both domestically and abroad. Internationally recognized research guidelines, such as Good Laboratory Practice (GLP) and Good Clinical Practice (GCP), are followed in all pre-clinical and clinical investigations.
Sanostem has collaborations with researchers and physicians from prestigious universities, top research institutes, and recognized healthcare facilities both domestically and abroad. Internationally recognized research guidelines, such as Good Laboratory Practice (GLP) and Good Clinical Practice (GCP), are followed in all pre-clinical and clinical investigations.

Strategy
By adhering to rigorous international research guidelines like GLP and GCP and collaborating with prestigious universities and top-tier institutions both domestically and internationally, SanoStem is paving the way for innovative stem cells based therapeutic solutions where none currently exist. This kind of work has the potential to make a significant impact on medical science and patient care.

Sponsored by Sanostem Cell Technologies, Inc.’s licensee CardioCell, LLC.
