Mesenchymal stem cells in the treatment of severe COVID-19
Abstract
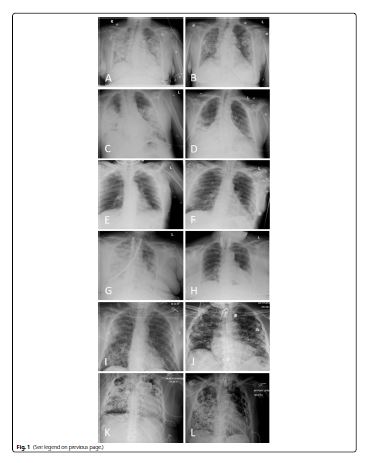
Here, we report a prospective case series of patients with laboratory confirmed COVID-19 treated with allogeneic bone marrow-derived, ischemic tolerant hypoxic MSCs (Stemedica Cell Technology, San Diego, CA USA) at two hospitals in the United States. Te data represents nine patients treated under emergency use IND and five treated under an expanded use IND at ProMedica Toledo Hospital (Toledo, OH USA) and Providence Saint John’s Health Center (Santa Monica, CA USA). All patients were categorized as severe or critically severe on admission, as previously defined [6]. Patient populations were somewhat dissimilar as shown in Table 1. Those treated under the expanded use were more severely ill, more often failed other potential COVID-19 treatments, and were infused with MSCs later in their clinical course. All expanded use and five emergency use cases were treated with 50× 106 cells twice, on average 5 days apart. Four emergency use patients were given a single bolus of 100× 106 cells, one of whom received approximately 64 M cells. Cells were delivered as an infusion in a central or peripheral line in an upper extremity as a constant rate of 2 M cells/minute. Dose per kg of body weight varied from 0.42× 106 cells/kg to 1.5× 106 cells/kg, 0.69× 106 cells/kg on average. Patients were tested for allergic skin reactions with subcutaneous microdose prior to infusion.
| All patients (n = 14) | Emergency use (n = 9) | Expanded use (n = 5) | |
|---|---|---|---|
| Median age – years (range) | 58.5 (30 – 84) | 64 (56 – 84) | 38.0 (30 – 59) |
| Female sex – n (%) | 6 (42.9) | 4 (44.4) | 2 (40) |
| Comorbid conditions | |||
| Cardiovascular disease – n (%) | 1 (7.1) | 1 (11.1) | 0 (0) |
| COPD – n (%) | 4 (28.6) | 4 (44.4) | 0 (0) |
| Hypertension – n (%) | 7 (50) | 7 (77.8) | 0 (0) |
| Obesity – n (%) | 8 (57.1) | 3 (33.3) | 5 (100) |
| Other pulmonary disease – n (%) | 6 (42.9) | 6 (66.6) | 0 (0) |
| Type II diabetes – n (%) | 3 (21.4) | 2 (22.2) | 1 (20) |
| COVID-19 Severity on admission | |||
| Severe – n (%) | 5 (35.7) | 5 (55.6) | 0 (0) |
| Critically severe – n (%) | 9 (64.3) | 4 (44.4) | 5 (100) |
| Days of admission prior to treatment with MSCs – median (range) | 10 (2 – 77) | 4 (2 – 12) | 41 (13 – 77) |
| Days between MSC treatments – median (range) | 5 (2 – 8) | 3 (2 – 3) | 7 (6 – 8) |
| Failed or concurrent therapies | |||
| Antibiotics | 14 (100) | 9 (100) | 5 (100) |
| Antiviral | 5 (35.7) | 1 (11.1) | 4 (80) |
| Convalescent serum | 6 (42.9) | 1 (11.1) | 5 (100) |
| Hydroxychloroquine | 8 (57.1) | 5 (55.6) | 3 (60) |
| Interleukin-6 inhibitor | 6 (42.9) | 1 (11.1) | 5 (100) |
| Steroids | 10 (71.4) | 8 (88.9) | 2 (40) |
| Oxygen therapy required | |||
| Mechanical ventilation | 10 (71.4) | 5 (55.6) | 5 (100) |
| ECMO | 5 (35.7) | 0 (0) | 5 (100) |
| Pneumonia | 13 (92.9) | 8 (88.9) | 5 (100) |
| Concurrent bacterial pneumonia | 7 (50) | 2 (22.2) | 5 (100) |
| ARDS | 9 (64.3) | 4 (44.4) | 5 (100) |
| LOS post-treatment | 15 (2 – 50) | 13 (2 – 50) | 17 (15 – 25) |
| Total length of stay daysb | 31.5 (5 – 98) | 17 (5 – 63) | 71 (45 – 98) |
| Survival – post treatment with MSCs | |||
| 7 days | 13 (92.9) | 8 (88.9) | 5 (100) |
| 14 days | 12 (85.7) | 7 (77.8) | 5 (100) |
| 21 days | 12 (85.7) | 7 (77.8) | 5 (100) |
| 30 days | 9 (64.3) | 7 (77.8) | 2 (40) |
We thank Will and Cary Singleton for support of the Pacific Neuroscience Institute Stem Cell Program and Stemedica for providing cells.
Ethics approval and consent to participate
Lev Verkh and Nikolai Tankovich are employees of Stemedica, which provided the stem cells used in this report.
2. Tyndall A, van Laar JM. Stem cell transplantation and mesenchymal cells to treat autoimmune diseases. Presse Med. 2016;45(6 Pt 2):e159–69.
3. Lin F, Ichim TE, Pingle S, et al. Mesenchymal stem cells as living antiinfammatory therapy for COVID-19 related acute respiratory distress syndrome. World J Stem Cells. 2020;12(10):1067–79.
4. Moll G, Drzeniek N, Kamhieh-Milz J, et al. MSC therapies for COVID-19: importance of patient coagulopathy, thromboprophylaxis, cell product quality and mode of delivery for treatment safety and efcacy. Front Immunol. 2020;11:1091.
5. Taghavi-Farahabadi M, Mahmoudi M, Soudi S, Hashemi SM. Hypothesis for the management and treatment of the COVID-19-induced acute respiratory distress syndrome and lung injury using mesenchymal stem cell-derived exosomes. Med Hypotheses. 2020;144:109865.
6. Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–28.
7. Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33.
